The U.S. Food and Drug Administration (FDA) recently made a key regulatory move by determining 35 PFAS food contact notifications no longer effective. This is because the relevant companies have informed the FDA in writing that they have ceased the production, supply, or use of the listed food contact substances within the U.S. This marks a further advancement in the FDA’s regulatory efforts to completely ban the use of PFAS-containing food contact materials in paper and paperboard products.
PFAS, or Per and Polyfluoroalkyl Substances, are widely used due to their non-stick and grease, oil, and water-resistant properties.
The FDA had previously authorized the following uses of PFAS:
1.Non-stick applications on pots & pans
2.Rubber O-rings & gaskets used in food processing equipment
3.Manufacturing aids added to other food contact polymers
4. Grease-Proofers applied to fast-food wrappers, microwave popcorn bags, take-out paperboard containers, and pet food bags.
Based on the data currently available to the FDA, oil repellents in paper and paperboard can cause PFAS to migrate into food, potentially creating safety hazards. In 2024, after consulting with relevant manufacturers, the FDA confirmed that PFAS-containing substances would no longer be sold as oil repellents (item 4 above) in the U.S. market. According to the FDA’s January 6, 2025, Federal Register publication, these authorizations no longer be effective.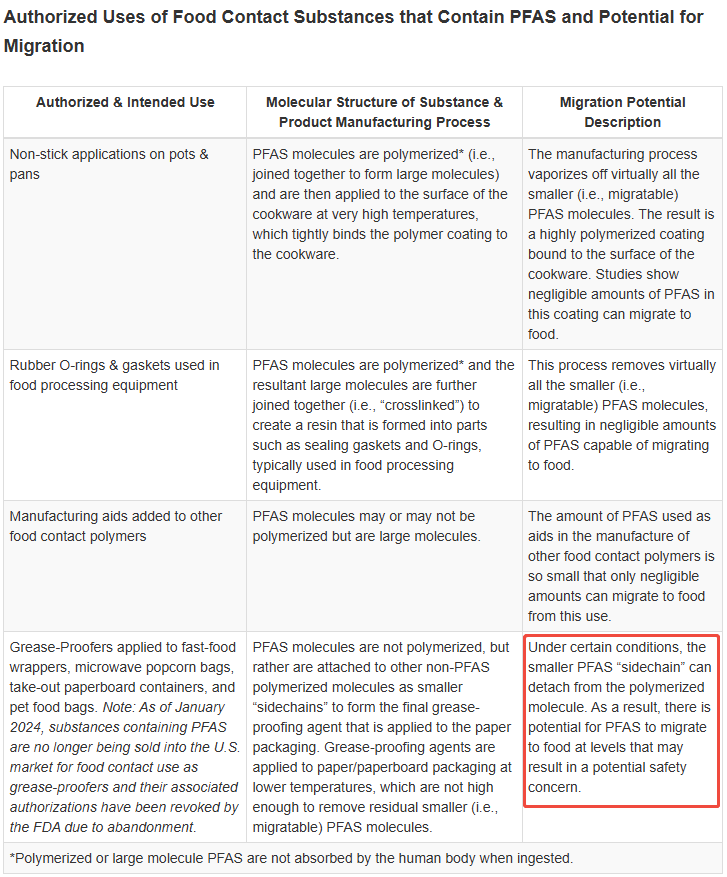
Timeline:
2020: The FDA conducted a post-market safety assessment and found potential safety concerns related to 6:2 Fluorotelomer Alcohol (6:2 FTOH), a short-chain PFAS. Following this, the FDA announced voluntary agreements with several companies to phase out specific types of PFAS used in food packaging.
2024: The FDA confirmed that PFAS used in grease-resistant food packaging are no longer being sold by manufacturers for food contact use in the U.S. market, completing the phase-out plan initiated in 2020.
2025: The FDA published a notice in the Federal Register announcing that 35 Food Contact Notifications for PFAS substances used as grease-proofing agents in paper and paperboard food packaging are no longer effective.
As an internationally recognized third-party testing and certification organization, BTF offers comprehensive food testing services and assistance with U.S. FDA food facility registration. We welcome your inquiries!
BTF Testing Lab, our company has electromagnetic compatibility laboratories, safety regulations Laboratory, wireless radio frequency Laboratory, battery Laboratory, chemical Laboratory, SAR Laboratory, HAC Laboratory, etc. We have obtained qualifications and authorizations such as CMA, CNAS, A2LA, CPSC, VCCI, etc. Our company has an experienced and professional technical engineering team, which can help enterprises solve the problem. If you have relevant testing and certification needs, you can directly contact our Testing staff to obtain detailed cost quotations and cycle information!
Post time: May-13-2025